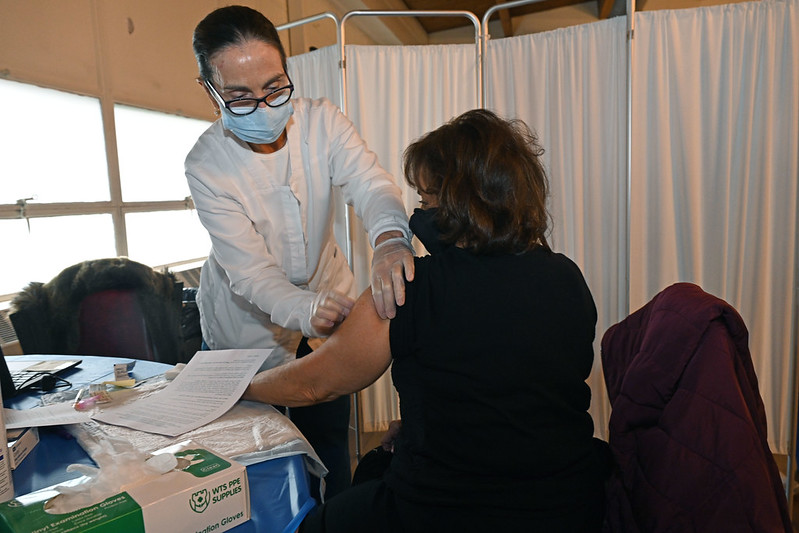
Cuomo said that adults of any age with the following conditions will be eligible:
- Cancer (current or in remission, including 9/11-related cancers)
- Chronic kidney disease
- Pulmonary Disease, including but not limited to, COPD (chronic obstructive pulmonary disease), asthma (moderate-to-severe), pulmonary fibrosis, cystic fibrosis, and 9/11 related pulmonary diseases
- Intellectual and Developmental Disabilities including Down Syndrome
- Heart conditions, including but not limited to heart failure, coronary artery disease, cardiomyopathies, or hypertension (high blood pressure)
- Immunocompromised state (weakened immune system) including but not limited to solid organ transplant or from blood or bone marrow transplant, immune deficiencies, HIV, use of corticosteroids, use of other immune weakening medicines, or other causes
- Severe Obesity (BMI 40 kg/m2), Obesity (body mass index [BMI] of 30 kg/m2 or higher but < 40 kg/m2)
- Pregnancy
- Sickle cell disease or Thalassemia
- Type 1 or 2 diabetes mellitus
- Cerebrovascular disease (affects blood vessels and blood supply to the brain)
- Neurologic conditions including but not limited to Alzheimer’s Disease or dementia
- Liver disease
Cuomo also backed up his health commissioner, Dr. Howard Zucker, who on Thursday rebuffed Mayor Bill de Blasio’s request to tap into the second dose reserve to administer more first doses to unvaccinated New Yorkers. The governor said the state lacked the authority to do that anyway, and is following federal guidance on how the vaccines are administered.
“The FDA has spoken specifically to this,” Cuomo said, adding that “without appropriate data, we run the significant risk of placing the public health at risk” and undermining all vaccination efforts.
But the governor indicated that he didn’t completely rule out the second dose idea.
“The federal position is clear. I have spoken to them about using a percentage of the second dose as the first dose. It’s complicated, but I think it could be done,” the governor added. “The federal government is not there at this point. They’ve not approved it. But if they do approve it, then New York is ready, willing and able to do it. But it’s a federal decision.”
The question of using the second dose supply as the first dose could be rendered almost moot if the federal government gives emergency approval to the Johnson & Johnson COVID-19 vaccine.
Cuomo called the one-shot formula a potential “game-changer” which could prompt an immediate boost in vaccine supply; Johnson & Johnson has indicated it could have 100 million doses ready and distributed by June, should it receive emergency FDA approval.
So far, with the two-dose Pfizer and Moderna vaccines in use, more than 2.2 million New Yorkers have been vaccinated, with 495,000 of them having received the second dose. New York has used 99.02% of its dosages, and is now waiting for next week’s allocation, according to Cuomo.
The state continues to work to expand usage of the vaccine among Black and Latino New Yorkers. Cuomo cited data gathered through the first round vaccinations which indicated that not as many Black New Yorkers had received the shot.
Cuomo said the state is working with community groups to shake off cynicism surrounding the COVID-19 vaccine and encourage all New Yorkers, regardless of color and background, to get the shot when it is available. An ad campaign is also planned.
“There are bonafide reasons for distrust of the system, I get it. But it’s not true with this vaccine, and this is going to be a process of communication and we’re going to have to talk through it,” the governor concluded.
For more coronavirus coverage, visit longislandpress.com/coronavirus.
Sign up for Long Island Press’ email newsletters here. Sign up for home delivery of Long Island Press here. Sign up for discounts by becoming a Long Island Press community partner here.