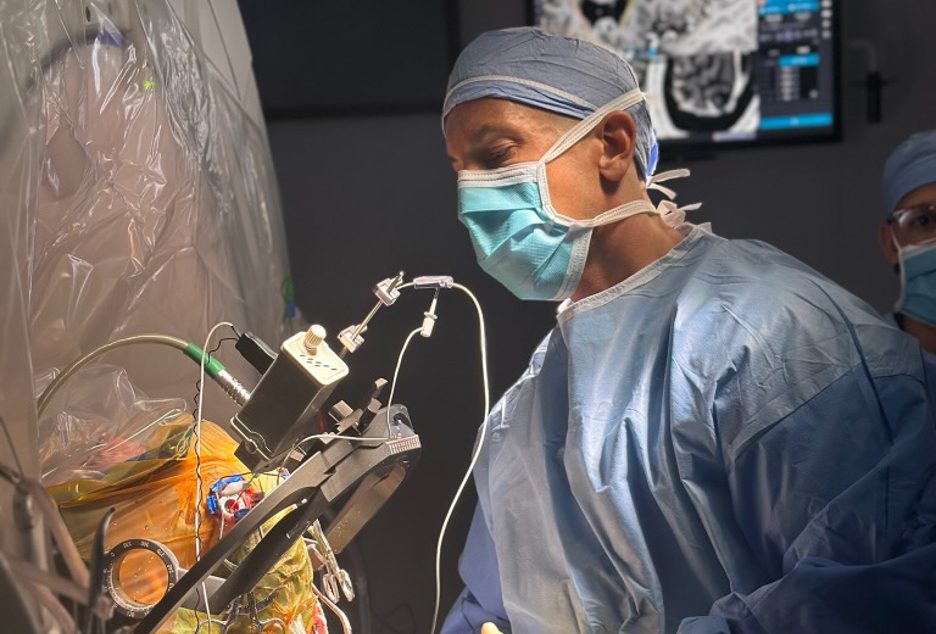
Northwell Health is conducting a clinical trial to address treatment-resistant depression using similar technology used to treat neurological diseases like Parkinson’s in an effort to seek options for individuals who have exhausted all others.
“If we can help these patients and get them back in the workforce, get them back to live a life that they enjoy, back to being fathers or mothers or family members, that’s huge,” said Dr. Albert Fenoy, who is leading the clinical trial. “And we’ve seen that. These patients go back to work, go back to being a family person. It’s tremendous.”
Fenoy, director of functional neurosurgery at Northwell, is the clinical trial’s lead investigator. He originally began the research in 2014 with a clinical trial at the University of Texas at Houston, where Fenoy used to work.
He is continuing the trial at Northwell Health, which is funded by a $3 million grant from the National Institute of Health.
The clinical trial studies patients with treatment-resistant depression and the effects of electrodes implanted in the brain in reducing symptoms.
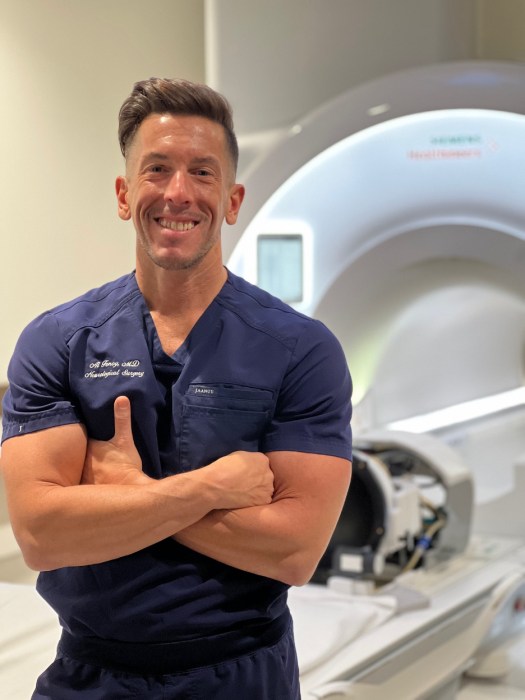
Fenoy said patients who participate in the trial do not experience pleasure to the same degree that a typical patient may. He called this condition anhedonia.
The procedure involves implanting electrodes into the part of the brain that contributes to the body’s reward network called the medial forebrain bundle. This procedure is called deep brain stimulation.
The electrodes stimulate the brain’s reward system to increase the patient’s ability to experience pleasure, which he said, in turn, can improve the patient’s depression.
Once the electrodes are implanted, they may not be turned on immediately.
Fenoy said that due to the subjectivity of the disease, patients are not told when the electrodes are turned on so as not to yield false results. Instead, the electrodes are turned on and off randomly to objectively observe the effects on the patient.
While brain surgery sounds daunting, Fenoy said it is less scary than it sounds. He said many patients just stay overnight in the hospital after the procedure before going home the next day and resuming their life.
Treatment-resistant depression is not a rarity, with about one-third of adults with major depression with symptoms that aren’t alleviated with standard treatments, according to Northwell Health.
“There’s a great need for this,” Fenoy said. “It’s a tremendous need and we haven’t come to it in our society yet to figure out how we can help these patients where every other treatment measure fails.”
Fenoy’s first clinical trial, which ran from 2014 to 2022, yielded promising results. Eleven of the 14 participants displayed a 50% improvement in their depression symptoms, while the other three showed a 30% improvement.
Gregorio Lozano, 44, was one of Fenoy’s patients during his Texas clinical trial. He underwent the deep brain stimulation procedure in 2015, which he reported aiding depression and anxiety symptoms he had been experiencing since middle school.
“My depression has pretty much been eradicated,” Lozano, who still has electrodes implanted in his brain, said in a release.
While Lozano still takes medication for depression, he said it is significantly reduced compared to his prior prescription cocktail.
“So those are over 10 years that we’ve had patients without depression,” Fenoy said.
Fenoy said the trial at Northwell Health will continue the work he began in Texas, using more imaging to foster a more detailed study.
The clinical trial is FDA-approved, but the deep brain stimulation procedure for treating depression is not. Fenoy said the purpose of continuing the clinical trial is to study the procedure with more patients to work towards understanding the treatment’s efficacy “beyond a doubt.”
“We need to prove undoubtedly that what we’re doing as an intervention is absolutely going to help,” Fenoy said. “And that requires a lot of patients and a very well-designed trial.”
While this treatment has been studied globally over the past decade, Fenoy estimated less than 500 patients have undergone the procedure for treatment-resistant depression. He said this is not enough.
Fenoy said his goal is to recruit another 20 patients over the next five years. He said they are still actively recruiting patients to participate in the five-year clinical trial.